Dr. V.K.Maheshwari, M.A. (Socio, Phil) B.Sc. M. Ed, Ph.D. Former Principal, K.L.D.A.V.(P.G) College, Roorkee, India
Mrs Sudha Rani Maheshwari, M.Sc (Zoology), B.Ed. Former Principal. A.K.P.I.College, Roorkee, India
“The size of this year’s ozone hole is approaching an all-time high, but it will probably not break any records.” Geir Braathen
Ozone is a colorless gas found in the upper atmosphere of the Earth. It is formed when oxygen molecules absorb ultraviolet photons, and undergo a chemical reaction known as photo dissociation or photolysis. In this process, a single molecule of oxygen breaks down into two oxygen atoms. The free oxygen atom (O), then combines with an oxygen molecule (O2), and forms a molecule of ozone (O3). The ozone is located between 4 and 10 miles above the surface of the Earth (lower near the poles), with the highest ozone concentrations in a region that is variously called the lower stratosphere, the tropopause, or simply the “ozone layer”.
Ozone itself is a diatomic molecule, composed of three oxygen atoms that bonded, unlike the oxygen we breathe, which are diatomic molecules, meaning two oxygen atoms. The “ozone layer” contains more than 90% of the earth’s ozone. Ozone is a corrosive, light blue gas with a smell something like burning electrical wiring. The atmosphere at this altitude is still about 78% nitrogen, 21% oxygen, and the peak ozone concentration is about 9 pm (or 0.0009%). Other things (water vapour, carbon dioxide, argon, and so on) are present there in small concentrations too.
The ozone molecules, in turn absorb ultraviolet rays between 310 to 200 nm (nanometers) wavelength, and thereby prevent these harmful radiations from entering the Earth’s atmosphere. The process of absorption of harmful radiation occurs when ozone molecules split up into a molecule of oxygen, and an oxygen atom. The oxygen atom (O), again combines with the oxygen molecule (O2) to regenerate an ozone (O3) molecule. Thus, the total amount of ozone is maintained by this continuous process of destruction, and regeneration.
Meaning of Ozone Depletion
The ozone layer is a thin layer of ozone that protects the earth from dangerous ultraviolet light. Destruction of the stratospheric ozone layer which shields the earth from ultraviolet radiation harmful to life. This destruction of ozone is caused by the breakdown of certain chlorine and/or bromine containing compounds (chlorofluorocarbons or halogens), which break down when they reach the stratosphere and then catalytically destroy ozone molecules.
If you took all the ozone in an entire column from the ground to infinity, and compressed it to STP (standard temperature and pressure, or 0°C at 1 atmosphere pressure) it would be a layer about 3 mm thick. There is less than 1/3 as much in the Antarctic “ozone hole” when it is winter there
The ozone layer is a region high in the stratosphere, containing ozone (a form of oxygen) that filters out most of the Sun’s dangerous ultraviolet rays (UV-B). This UV-B could otherwise be absorbed by the DNA in all surface dwelling life on Earth.
Destruction of the stratospheric ozone layer which shields the earth from ultraviolet radiation harmful to life. This destruction of ozone is caused by the breakdown of certain chlorine and/or bromine containing compounds (chlorofluorocarbons or halons), which break down when they reach the stratosphere
The degradation of the Earth`s protective layer of ozone in the high atmosphere by some industrial and domestic gases.
Ozone depletion describes two distinct but related phenomena observed since the late 1970s : a steady decline of about 4% per decade in the total volume of ozone in Earth`s stratosphere (the ozone layer), and a much larger springtime decrease in stratospheric ozone over Earth`s polar regions.
There are two different types of ozone depletion, both are very similar. The first one has been a slow, but steady ozone depletion of 4% per decade of the Earth’s stratosphere(ozone layer). This has been happening constantly since the 1970′s. The other is a much larger, although seasonal loss of ozone over the polar regions. This yearly occurrence is called the ozone hole. There are many causes for ozone depletion, but the most important process in both trends is catalytic destruction of ozone by atomic chlorine and bromine. Both come from the breaking down of chloroflourocarbons by photons in the atmosphere.
Meaning of Ozone Hole
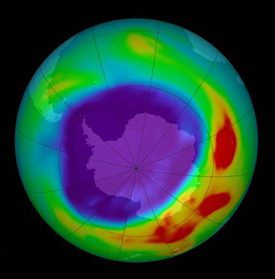
An ozone hole also periodically forms, since ozone is unstable. The word “hole” is somewhat misleading. This is actually a diminished concentration of ozone due to the lack of sunlight and not a complete absence. An ozone hole forms over a pole, then later closes, once each year at the pole that is not receiving UV-C light from the Sun. The southern polar hole is larger than the northern polar hole, due to the polarity of Earth’s magnetic field. Chlorofluorocarbons (CFCs) make the hole larger, last longer, and contain less ozone, which is only an indication of the general “health” of the ozone layer. The hole itself forms in areas that are receiving no UV-B from the Sun either, so there are no life forms at risk from our Sun directly “beneath the hole,” but every surface organism is at risk from a thinned ozone layer.
Effect of Ozone Layer Depletion
A .Effects on Human and Animal Health
Increased penetration of solar UV-B radiation is likely to have profound impact on human health with potential risks of eye diseases, skin cancer and infectious diseases . UV radiation is known to damage the cornea and lens of the eye. Chronic exposure to UV-B could lead to cataract of the cortical and posterior subcapsular forms. UV-B radiation can adversely affect the immune system causing a number of infectious diseases. In light skinned human populations, it is likely to develop nonmelanoma skin cancer (NMSC). Experiments on animals show that UV exposure decreases the immune response to skin cancers, infectious agents and other antigens
B. Effects on Terrestrial Plants
It is a known fact that the physiological and developmental processes of plants are affected by UV-B radiation. Scientists believe that an increase in UV-B levels would necessitate using more UV-B tolerant cultivar and breeding new tolerant ones in agriculture. In forests and grasslands increased UV-B radiation is likely to result in changes in species composition (mutation) thus altering the bio-diversity in different ecosystems . UV-B could also affect the plant community indirectly resulting in changes in plant form, secondary metabolism, etc. These changes can have important implications for plant competitive balance, plant pathogens and bio-geochemical cycles.
C. Effects on Aquatic Ecosystems
While more than 30 percent of the world’s animal protein for human consumption comes from the sea alone, it is feared that increased levels of UV exposure can have adverse impacts on the productivity of aquatic systems. High levels of exposure in tropics and subtropics may affect the distribution of phytoplanktons which form the foundation of aquatic food webs. Reportedly a recent study has indicated 6-12 percent reduction in phytoplankton production in the marginal ice zone due to increases in UV-B. UV-B can also cause damage to early development stages of fish, shrimp, crab, amphibians and other animals, the most severe effects being decreased reproductive capacity and impaired larval development.
D. Effects on Amphibians
Ozone depletion is listed as one of the causes for the declining numbers of amphibian species. Ozone depletion affects many species of amphibians at every stage of their life cycle. Some of the effects are mentioned below.
- Hampers growth and development in larvae
- Changes behaviour and habits
- Causes deformities in some species
- Decreases immunity. Some species have become more vulnerable to diseases and death
- Retinal damage and blindness in some species
E. Effects on Marine Ecosystems
In particular, plankton (phytoplankton and bacterioplankton) are threatened by increased UV radiation. Marine phytoplankton play a fundamental role in both the food chain as well as the oceanic carbon cycle. Plankton plays an important role in converting atmospheric carbon dioxide into oxygen. Ultraviolet rays can influence the survival rates of these microscopic organisms, by affecting their orientation and mobility. This eventually disturbs and affects the entire ecosystem.
F. Effects on Bio-geo-chemical Cycles
Increased solar UV radiation could affect terrestrial and aquatic bio-geo-chemical cycles thus altering both sources and sinks of greenhouse and important trace gases, e.g. carbon dioxide (CO2), carbon monoxide (CO), carbonyl sulphide (COS), etc. These changes would contribute to biosphere-atmosphere feedbacks responsible for the atmosphere build-up of these gases. Other effects of increased UV-B radiation include: changes in the production and decomposition of plant matter; reduction of primary production changes in the uptake and release of important atmospheric gases; reduction of bacterioplankton growth in the upper ocean; increased degradation of aquatic dissolved organic matter (DOM), etc. Aquatic nitrogen cycling can be affected by enhanced UV-B through inhibition of nitrifying bacteria and photodecomposition of simple inorganic species such as nitrate. The marine sulphur cycle may also be affected resulting in possible changes in the sea-to-air emissions of COS and dimethylsulfied (DMS), two gases that are degraded to sulphate aerosols in the stratosphere and troposphere, respectively.
G. Effects on Air Quality
Reduction of stratospheric ozone and increased penetration of UV-B radiation result in higher photo dissociation rates of key trace gases that control the chemical reactivity of the troposphere. This can increase both production and destruction of ozone and related oxidants such as hydrogen peroxide which are known to have adverse effects on human health, terrestrial plants and outdoor materials. Changes in the atmospheric concentrations of the hydroxyl radical (OH) may change the atmospheric lifetimes of important gases such as methane and substitutes of chlorofluoro carbons (CFCs). Increased troposphere reactivity could also lead to increased production of particulates such as cloud condensation nuclei from the oxidation and subsequent nucleation of sulphur of both anthropogenic and natural origin (e.g. COS and DMS).
H. Effects on Materials
An increased level of solar UV radiation is known to have adverse effects on synthetic polymers, naturally occurring biopolymers and some other materials of commercial interest. UV-B radiation accelerates the photo degradation rates of these materials thus limiting their lifetimes. Typical damages range from discoloration to loss of mechanical integrity. Such a situation would eventually demand substitution of the affected materials by more photo stable plastics and other materials in future. The chlorine radicals thus produced can undergo complex chemical reaction producing chlorine monoxide which can attack an ozone molecule converting it into oxygen and in the process regenerating the chlorine atom again. Thus the ozone destroying effect is catalytic and a small amount of CFC would be destroying large number of ozone molecules
I. Effects on Climate Change
Ozone depletion and climate change are linked in a number of ways, but ozone depletion is not a major cause of climate change. Atmospheric ozone has two effects on the temperature balance of the Earth. It absorbs solar ultraviolet radiation, which heats the stratosphere. It also absorbs infrared radiation emitted by the Earth’s surface, effectively trapping heat in the troposphere. Therefore, the climate impact of changes in ozone concentrations varies with the altitude at which these ozone changes occur. The major ozone losses that have been observed in the lower stratosphere due to the human-produced chlorine- and bromine-containing gases have a cooling effect on the Earth’s surface. On the other hand, the ozone increases that are estimated to have occurred in the troposphere because of surface-pollution gases have a warming effect on the Earth’s surface, thereby contributing to the “greenhouse” effect. In comparison to the effects of changes in other atmospheric gases, the effects of both of these ozone changes are difficult to calculate accurately.
J. Effects on Ultraviolet Radiation
The depletion of the ozone layer leads, on the average, to an increase in ground-level ultraviolet radiation, because ozone is an effective absorber of ultra-violet radiation. The Sun emits radiation over a wide range of energies, with about 2% in the form of high-energy, ultraviolet (UV) radiation. Some of this UV radiation (UV-B) is especially effective in causing damage to living beings, the largest decreases in ozone during the past 15 years have been observed over Antarctica, especially during each September and October when the ozone hole forms.In areas where smaller ozone depletion has been observed, UV-B increases are more difficult to detect. In particular, detection of trends in UV-B radiation associated with ozone decreases can be further complicated by changes in cloudiness, by local pollution, and by difficulties in keeping the detection instrument in precisely the same condition over many years.
International Remedial Actions
Ozone was discovered by C.F. Schonbein in 1840. In the lower atmosphere O3 is a Pollutant and is produced by Photochemical reactions involving sunlight, Nitrogen Oxides, Hydrocarbons and diatomic oxygen in the stratosphere, O3 provides essential shield against damaging Ultra violet radiation. Approximately 90% of the Ozone in the atmosphere is found in the stratosphere, where peak concentrations are about 300 PPb. The ozone layer in the Stratosphere is often called the ozone shield, because it absorbs most Ultra Violet radiation that is potentially damaging to life. The Ozone layer was discovered in 1913 by the French Physicist Charles Fabry and Henry Buisson. its properties were explored in detail by the British meteorologist GMB Dobson, who developed a simple spectrophotometer (the dosimeter) that could be used to measure Spectrophotometer ozone from the ground.
The first international action to focus attention on the dangers of ozone depletion in the stratosphere and its dangerous consequences in the long run on life on earth was focused in 1977 when in a meeting of 32 countries in Washington D.C. a World plan on action on Ozone layer with UNEP as the coordinator was adopted.
Montreal Protocol
As experts began their investigation, data piled up and in 1985 in an article published in the prestigious science journal, “Nature” by Dr. Farman pointed out that although there is overall depletion of the ozone layer all over the world, the most severe depletion had taken place over the Antarctica. This is what is famously called as “the Antarctica Ozone hole”. His findings were confirmed by Satellite observations and offered the first proof of severe ozone depletion and stirred the scientific community to take urgent remedial actions in an international convention held in Vienna on March 22, 1985. This resulted in an international agreement in 1987 on specific measures to be taken in the form of an international treaty known as the Montreal Protocol on Substances That Deplete the Ozone Layer. Under this Protocol the first concrete step to save the Ozone layer was taken by immediately agreeing to completely phase out chlorofluorocarbons (CFC), Halogens, Carbon tetrachloride (CTC) and Methyl chloroform (MCF) as per a given schedule
In 1985 the Vienna Convention established mechanisms for international co-operation in research into the ozone layer and the effects of ozone depleting chemicals (ODCs). 1985 also marked the first discovery of the Antarctic ozone hole.
The treaty provides a time table on which the production of those substances must be phased out and eventually eliminated.
| Ozone depleting substances | Developed Countries | Developing Countries |
| CFC’s | Phased out end of 1995 | Total Phase out by 2010 |
| Halogens’ | Phased out end of 1993 | Total phase out by 2010 |
| Carbon tetrachloride | Phased out end of 1995 | Total phase out by 2015 |
| Methyl Chloroform | Phased out end of 1995 | Total phase out by 2015 |
| HCFCs | Total phase out by 2030 | Total phase out by 2015 |
| Methyl bromide | Total phase out by 2005 | Total phase out by 2015 |
On the basis of the Vienna Convention, the Montreal Protocol on Substances that Deplete the Ozone Layer was negotiated and signed by 24 countries and by the European Economic Community in September 1987. The Protocol called for the Parties to phase down the use of CFCs, halons and other man-made ODCs. The Montreal Protocol represented a landmark in the international environmentalist movement. For the first time whole countries were legally bound to reducing and eventually phasing out altogether the use of CFCs and other ODCs. Failure to comply was accompanied by stiff penalties. The original Protocol aimed to decrease the use of chemical compounds destructive to ozone in the stratosphere by 50% by the year 1999. The
Protocol was supplemented by agreements made in London in 1990 and in Copenhagen in 1992, where the same countries promised to stop using CFCs and most of the other chemical compounds destructive to ozone by the end of 1995.
Fortunately, it has been fairly easy to develop and introduce compounds and methods to replace CFC compounds. In order to deal with the special difficulties experienced by developing countries it was agreed that they would be given an extended period of grace, so long as their use of CFCs did not grow significantly. China and India, for example, are strongly increasing the use of air conditioning and cooling devices. Using CFC compounds in these devices would be cheaper than using replacement compounds harmless to ozone. An international fund was therefore established to help these countries introduce new and more environmentally friendly technologies and chemicals. The depletion of the ozone layer is a worldwide problem which does not respect the frontiers between different countries. It can only be affected through determined international co-operation.
In consequence, the Montreal Protocol has often been called the most successful international environment agreement to date.
Ozone depletion over India
With so much worry about the rapid ozone depletion taking place in various parts of the earth, Indian scientists are closely monitoring the ozone layer over India for possible depletion trends. Opinions are many and varied. According to S K Srivastava, head of the National Ozone Centre in New Delhi, there is no trend to show total ozone depletion over India. V.Thaphyal and S M Kulshresta of the Indian Meteorological Department also point out that for the period 1956 to 1986 “ozone measurements exhibit year to year variability, but do not show any increasing or decreasing trend over India.”
However, former director of the National Ozone Centre, K Chatterji, now with Development Alternatives, warns that there is no case for complacency. He asserts that his calculations exhibit an ozone depletion trend in the upper, layers of the stratosphere over New Delhi and Pune from 1980 to 1983 in the month of October when the Antarctic ozone hole is at its maximum. Since India already receives high doses of ultraviolet (UV-B) radiation, and is at the threshold go to speak, effects of ozone layer depletion could he far more disastrous in India. A P Mitra, former director general of the Council of Scientific and Industrial Research, clarifies that while there is no trend in the total ozone value, there is some evidence of ozone depletion at higher altitudes – at about 30 to 40 km – even over the tropics. He argues, however, that there is insufficient data and that the depletion may be due to solar cycles and other natural phenomena.
However, the effects of CFCs and belong cannot be ruled out. Total column ozone data has been recorded over India for a long time. A network of stations using Dobson spectrophotometers to mea- sure total ozone, some six times a day, covers Srinagar, New Delhi, Varanasi, Ahmadabad, Pone and Kodaikanal. Ozone profiles are also regularly using balloons. Ozone levels are the lowest during November and December and the highest in summer. Across the country, variations do exist. In Kodaikanal, the total ozone is 240 to 280 Dobson units (DU), in New Delhi 270 to 320 DU and in Srinagar 290 to 360 DU. One Dobson unit is the equivalent of 0.01 mm of compressed gas at a pressure of 760 rare mercury and 0°C.B. N. Srivastava of the National Physical Laboratory, who been working on incident UVradiation levels, says that during summer, at noon, the UV-B radiation with a wavelength of 290 manometer (nm) is equivalent to levels attained in the Antarctica during the ozone hole period. He warns that even a slight depletion of the ozone layer over India may lead to large percentage changes in UV-B radiation over the country. According to eminent skin specialists in New Delhi, the incidence of skin cancer in India is low, but they admit that the surveys conducted to identify any trends are inadequate. Controlled studies to observe the effects of changing UV- B radiation concentrations on crops are on, they said. However no field surveys have been done in the country as yet.